

- HOME
- ABOUT US
- NEWS
- FULL CYCLE GENETIC
TESTING SOLUTIONS - R&D GENETIC
TESTING SOLUTIONS - PATHOGENIC MICROBE GENETIC
TESTING SOLUTIONS - 中文

CLINICAL GENETIC TESTING SOLUTIONS
Full Cycle Cancer Management
By leveraging high-throughput sequencing technology and placing liquid biopsy at its core, HaploX offers a comprehensive range of clinical genetic testing solutions tailored to solid tumors, encompassing the entire cancer management cycle based on clinical requirements.
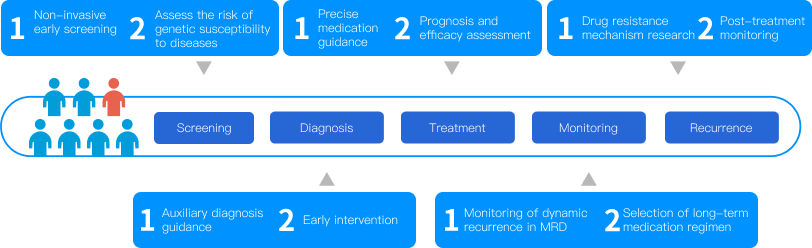
Pan-Cancer Genetic Testing Assays
Minimal Residual Disease (MRD) Detection Assays

HapOnco® mClear MRD Test

HapOnco® Lung Cancer mClean MRD Test

HapOnco® Gastrointestinal Cancer mClean MRD Test

HapOnco® Hepatobiliary and Pancreatic Cancer mClean MRD Test
Specific-Cancer Genetic Testing Assays

Lung Cancer

Gynecologic Cancer

Hepatobiliary and Pancreatic Cancer

Gastrointestinal Cancer

Breast Cancer

Urological Cancer
Service Commitments
Raw Data
Raw data is
available for
doctors and patients to conduct re-analysis.
Comprehensive
and Fast
Comprehensive assessment of tumor genetic information to guide clinical treatment, covering various sample
types; testing reports are issued within 7 to 10 days upon collecting qualified samples.
Professional Interpretation
Professional medical and genetic teams interpret the report, striving to maximize clinical benefits.
Anti-counterfeiting
Report
Each report is issued with a unique anti-counterfeiting QR code to verify its authenticity.
Self-developed Algorithms
The LIUDUS® platform, developed through in-house patented software, machine learning models and
internal data libraries, is applied throughout the entire NGS process, enabling ultra-sensitive variant
detection and providing physicians and patients with reliable clinical information.
Top-notch Quality
Control
Our laboratories have passed over 133 NCCL tests and certified by CAP (U.S.), CLIA (U.S.) and EMQN (EU).










