

- HOME
- ABOUT US
- NEWS
- FULL CYCLE GENETIC
TESTING SOLUTIONS - R&D GENETIC
TESTING SOLUTIONS - PATHOGENIC MICROBE GENETIC
TESTING SOLUTIONS - 中文

ONE-STOP SOLUTIONS
Genetic Testing One-stop Solutions
NGS wet lab solutions
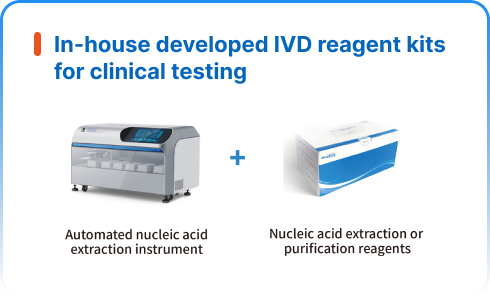
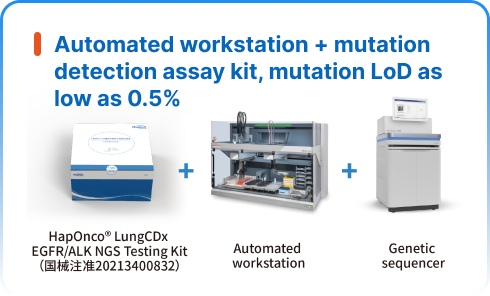
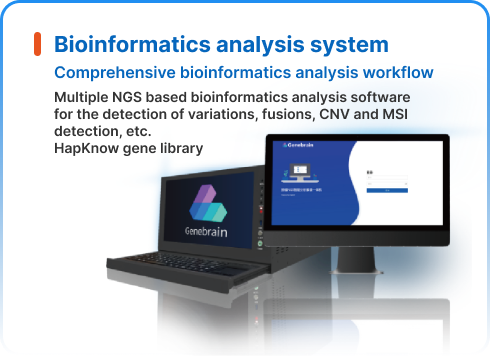
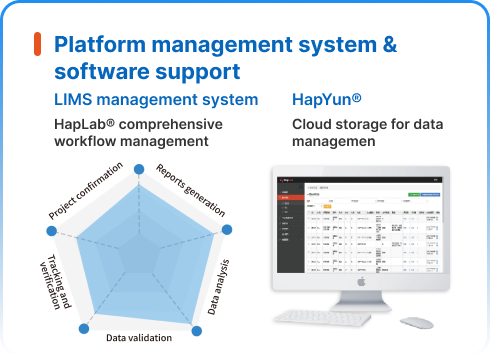
NGS dry lab solutions
Medical Devices
HaploX has a dedicated manufacturing facility that serves as the company's cornerstone for medical device research, production and registration. The manufacturing facility is designed in compliance with GMP requirements and accredited with ISO13485 certification, an internationally recognized quality control standard for the medical device industry. The HapOnco® LungCDx EGFR/ALK NGS Testing Kit has received Class III medical device registration certification from the National Medical Products Administration (NMPA). Currently, this kit is utilized for in vitro qualitative detection of non-small cell lung cancer (NSCLC) and has been widely promoted and used in targeted hospitals nationwide.





