- Home
- About Us
- Full Cycle Genetic
Testing Solutions - R&D Genetic
Testing Solutions - Pathogenic Microbe Genetic
Testing Solutions - Contact Us

HapOnco®
LungCDx EGFR/ALK NGS Testing Kit
HapOnco® LungCDx EGFR/ALK NGS Testing Kit
Product Overview
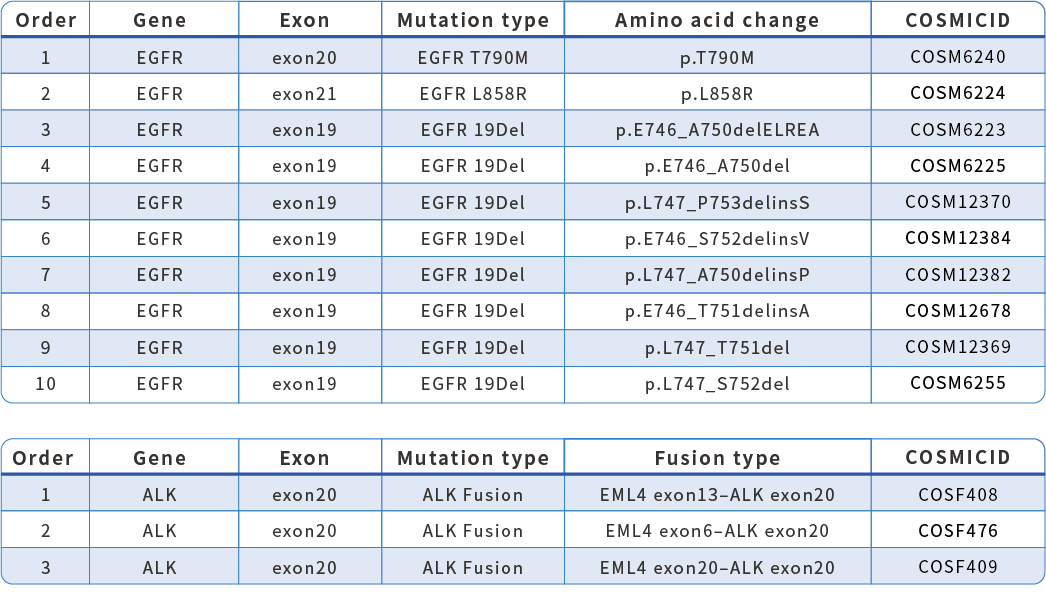
HapOnco® LungCDx EGFR/ALK NGS Testing Kit is an NGS-based therapy selection IVD candidate for NSCLC patients. This product was registered as a Class III medical device (国械注准20213400832) with the NMPA in October 2021. HapOnco® LungCDx detects EGFR/ALK gene mutations and has an industry-leading mutation LoD as low as 0.5% VAF, to identify patients eligible for treatment with multiple targeted therapies.
Product Information
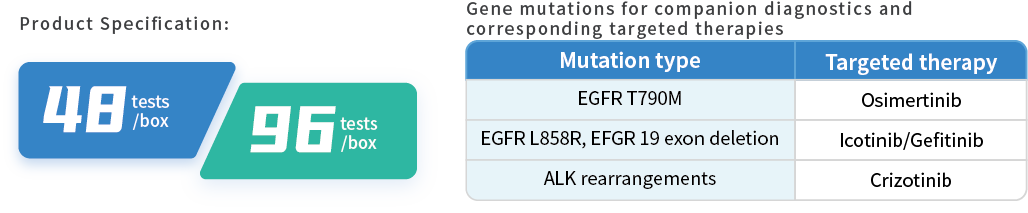
Product Performance
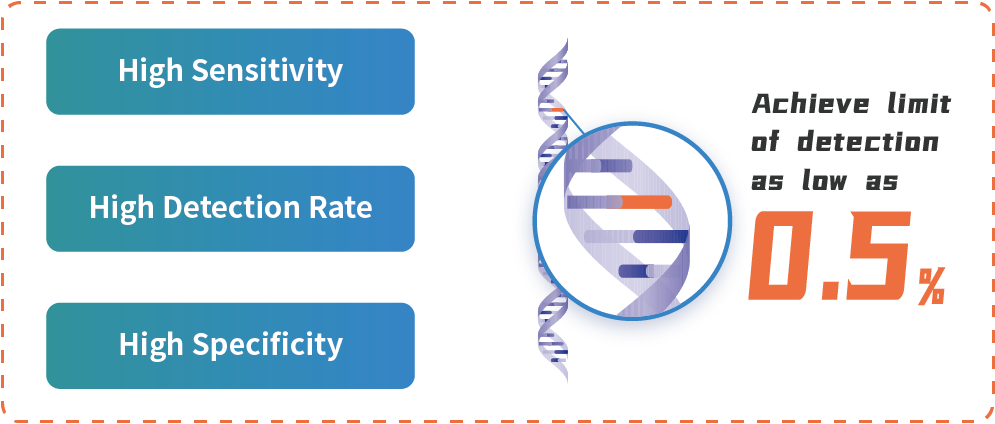
Product Advantages
HapOnco® LungCDx EGFR/ALK NGS Testing Kit is HaploX's NGS-based therapy selection IVD product for NSCLC with an industry-leading mutation LoD. It provides advanced companion diagnostics for cancer patients, further advancing precision medicine.

Mutations Detection