
- Home
- About Us
- Service
- Application
- Technology
- Resource
- Contact Us

HapOnco® Star
HapOnco® Star
Clinical 1326 - gene testing for solid tumors
Product Introduction
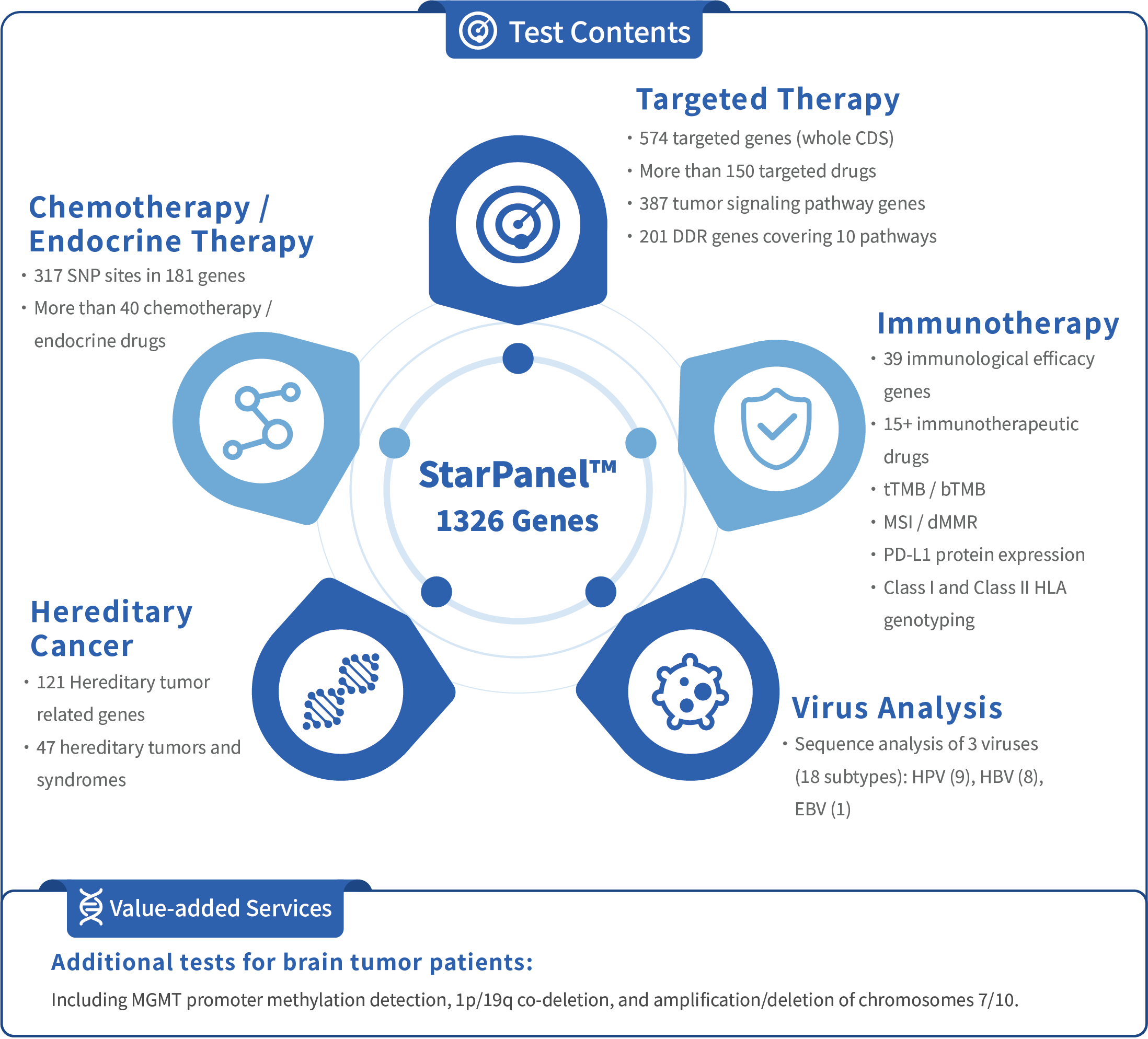
HapOnco®Star Panel Clinical 1326 - gene testing for solid tumors is a high - throughput sequencing product independently developed by HaploX for precision medicine in solid tumors. It can obtain important gene variation information related to tumor - targeted, immune, chemotherapy, endocrine therapy, hereditary tumors, and other cancer - related developments by testing tumor tissue, blood, malignant effusion, and other samples from patients. It also provides 18 - type detection of three viruses: HBV, HPV, and EBV. It offers reliable reference information for precise diagnosis and treatment by clinicians, helps in the precise management of the entire disease course for tumor patients, and maximizes clinical benefits.
Product Advantages
(1)Superior gene panel design: HapOnco®Star Panel has a superior gene panel design, covering genes related to approved targeted drugs and targeted therapy, MSI/tTMB/bTMB and other immune - related tests, genes related to chemotherapy/endocrine therapy, hereditary tumor risk assessment, and virus typing detection, which better meets the needs of clinical testing.
(2)High - depth sequencing: 20,000X ctDNA ultra - high - depth sequencing can detect mutations as low as 0.3%, avoiding missed detections and false negatives, and more accurately detects genomic mutations to assist in the analysis of drug - resistance mechanisms and dynamic monitoring of disease progression.
Applicable Population
![]() Solid tumor patients requiring targeted therapy and chemotherapy/endocrine therapy
Solid tumor patients requiring targeted therapy and chemotherapy/endocrine therapy
![]() Solid tumor patients who have developed resistance or have poor efficacy and need to adjust their treatment regimen
Solid tumor patients who have developed resistance or have poor efficacy and need to adjust their treatment regimen
![]() Solid tumor patients requiring immunotherapy (especially immune checkpoint inhibitors)
Solid tumor patients requiring immunotherapy (especially immune checkpoint inhibitors)
![]() Solid tumor patients with a family history of cancer or those needing to determine if the tumor is hereditary
Solid tumor patients with a family history of cancer or those needing to determine if the tumor is hereditary
