
- Home
- About Us
- Service
- Application
- Technology
- Resource
- Contact Us

News and Events
HaploX's Two DNA Extraction Kits Achieve EU CE-IVDR Certification, Delivering Efficient and Compliant Solutions for Precision Medicine
2025-12-12
December 12, 2025 — HaploX, a global innovator in precision oncology, today announced a pivotal advancement with the CE IVDR certification of its foundational nucleic acid extraction kits: the HapEnrich® (Tissue) and HapEnrich® (cfDNA) kits. This achievement underscores the company’s commitment to global quality standards , ensuring the initial and most critical step of the next-generation sequencing (NGS) workflow meets the highest international regulatory bar for performance safety and traceability.
The certification strengthens HaploX's global network of partners with unparalleled confidence, providing a robust foundation to drive forward advanced research and diagnostics. It underscores the company's mission to accelerate the delivery of precise cancer care worldwide through fully integrated, regulatory-compliant technology solutions.
Core Architecture of the Solution: One Platform, Dual Pathways
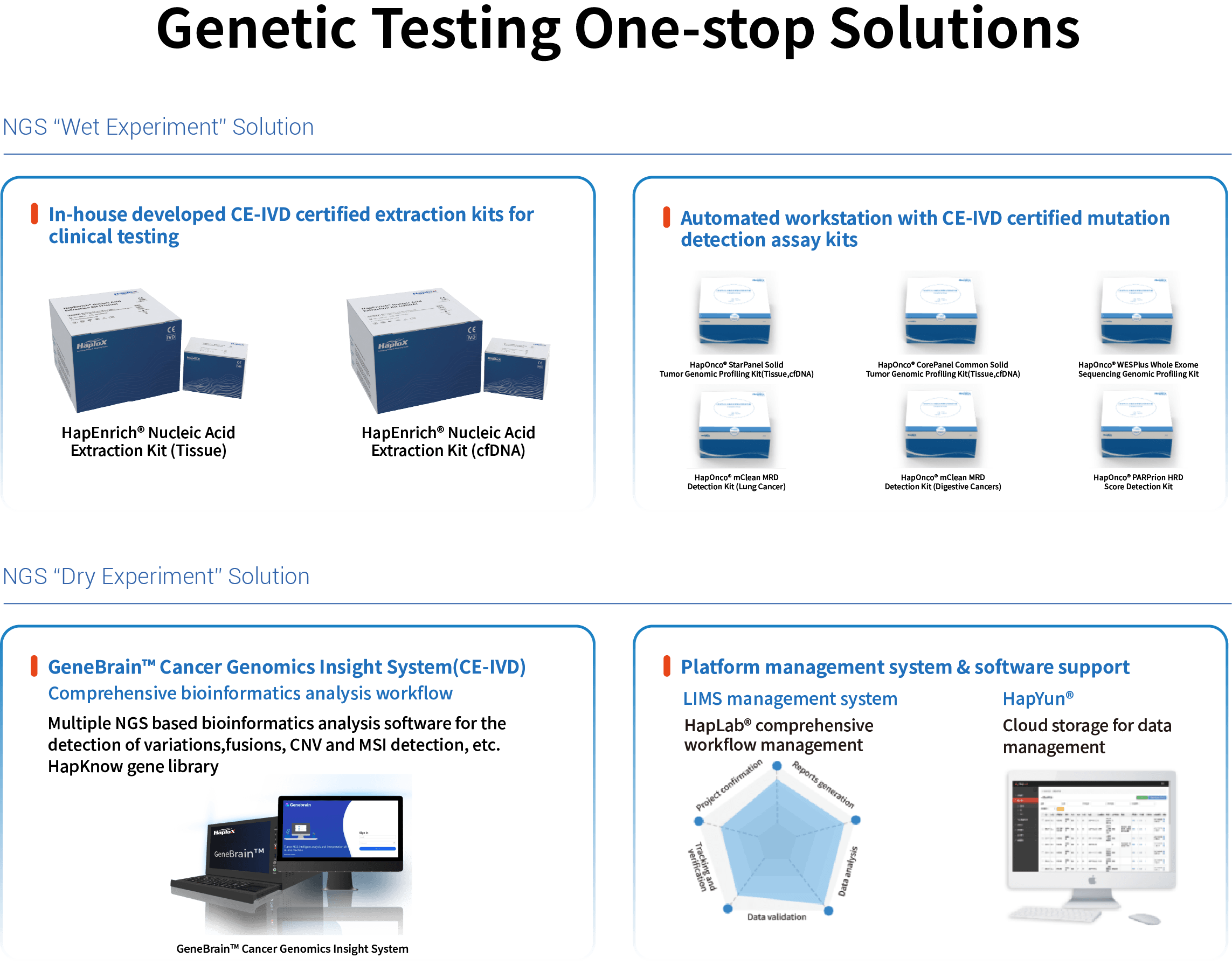
1、Complete Solution for Solid Tissue Samples — HapEnrich® (Tissue)
· Addresses Key Challenges: Specifically overcomes industry-wide difficulties such as severe DNA cross-linking, fragmentation, and low yield from FFPE samples.
· Application Scenarios: Provides high-quality genomic DNA for tumor tissue mutation testing, genetic disease analysis, pathogen identification, and more.
· Solution Value: Enables stable conversion from paraffin sections or tissue blocks to high-purity DNA, improving the success rate of tissue-based assays.

2、Dedicated Solution for Liquid Biopsy Samples — HapEnrich® (cfDNA)
· Addresses Key Challenges: Focuses on the technical hurdles of extremely low abundance, short fragment length, and susceptibility to background genomic DNA contamination in plasma cfDNA.
· Application Scenarios: Serves the entire lifecycle of liquid biopsy needs, including early cancer screening, dynamic therapy monitoring, and recurrence risk assessment.
· Solution Value: Efficiently enriches target fragment sizes (100-220 bp), maximizes the retention of low-frequency variant information, and paves the way for ultra-sensitive detection.

Strategic Advantages of the CE IVDR-Certified Workflow
The CE IVDR certification translates into tangible, operational benefits for laboratories worldwide, creating a seamless and trusted path from sample to sequencer.
| Advantage | Impact for Global Laboratories |
|---|---|
| Regulatory Confidence & Compliance | Provides a pre-verified, audit-ready starting point for IVD development and clinical testing, simplifying compliance in regulated markets (EU, IVDR-aligned regions) and strengthening study data for global submissions. |
| Unmatched Input Quality | Delivers nucleic acid of exceptional purity and fidelity, directly enhancing NGS library complexity, improving sequencing efficiency, and reducing false-positive variant calls caused by input material artifacts. |
| Streamlined Integrated Workflow | The kits are seamlessly optimized for use with the HaploX Library Prep System, eliminating compatibility guesswork. This integration reduces hands-on time, minimizes protocol optimization, and ensures consistent, reproducible results across sites and operators. |
| Global Standardization Enabler | Establishes a uniform, high-performance standard for sample processing across decentralized laboratories, multi-center clinical trials, and companion diagnostic partnerships, ensuring data consistency and comparability on an international scale. |
HaploX underscores its commitment to advancing the global scientific community by delivering technology that meets the highest international standards. The company's core objective is to empower researchers and clinicians worldwide to pioneer and implement the next generation of precision oncology through rigorous, certified solutions.
